Hydroiodic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names Hydronium iodide | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEBI |
| ||
ChemSpider |
| ||
EC Number | 233-109-9 | ||
PubChem CID |
| ||
RTECS number | MW3760000 | ||
UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | HI(aq) | ||
Molar mass | 127.91 | ||
| Appearance | colorless liquid | ||
Odor | acrid | ||
Density | 1.70 g/mL, azeotrope (57% HI by weight) | ||
Boiling point | 127 °C (261 °F; 400 K) 1.03 bar, azeotrope | ||
Solubility in water | Aqueous solution | ||
| Hazards | |||
EU classification (DSD) (outdated) | Corrosive (C) | ||
R-phrases (outdated) | R34 | ||
S-phrases (outdated) | (S1/2), S26, S45 | ||
NFPA 704 |  0 3 0 ACID | ||
Flash point | Non-flammable | ||
| Related compounds | |||
Other anions | Hydrofluoric acid Hydrochloric acid Hydrobromic acid | ||
Related compounds | Hydrogen iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Hydroiodic acid (or hydriodic acid) is a highly acidic aqueous solution of hydrogen iodide (HI)
(concentrated solution usually 48 - 57% HI). It is the second strongest hydrohalic acid, after hydroastatic acid. Hydroiodic acid is a commonly used chemical reagent and is one of the strong acids that ionize completely in an aqueous solution. Concentrated hydroiodic acid has a pH of less than 0.[citation needed]
Contents
1 Reactions
1.1 Cativa process
1.2 Illicit uses
2 References
3 External links
Reactions
Hydroiodic acid readily reacts with oxygen in air, contributing to the deep colours associated with old samples;
- 4 HI + O2 → 2 H
2O + 2 I2
- HI + I2 → HI3
Like other halogens, hydroiodic acid will perform addition reactions with unsaturated hydrocarbons such as alkenes.
Cativa process
The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol.[1][2]
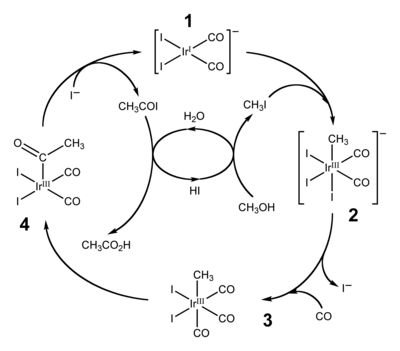
Illicit uses
Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from pseudoephedrine (recovered from nasal decongestant pills).[3] This reaction is stereospecific, producing only (d)-methamphetamine.
References
^ Jones, J. H. (2000). "The CativaTM Process for the Manufacture of Acetic Acid" (PDF). Platinum Metals Rev. 44 (3): 94–105..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Sunley, G. J.; Watson, D. J. (2000). "High productivity methanol carbonylation catalysis using iridium - The CativaTM process for the manufacture of acetic acid". Catalysis Today. 58 (4): 293–307. doi:10.1016/S0920-5861(00)00263-7.
^ Skinner, Harry F. "Methamphetamine Synthesis via HI/Red Phosphorus Reduction of Ephedrine". Forensic Science International, 48 128-134 (1990)
External links
- International Chemical Safety Card 1326
- European Chemicals Bureau



