1,1'-Bi-2-naphthol
| |||
| |||
| Names | |||
|---|---|---|---|
IUPAC name [1,1′-binaphthalene]-2,2′-diol | |||
| Other names 1,1′-bi-2-naphthol 1,1-binaphthol BINOL binol | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.009.104 | ||
PubChem CID |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C20H14O2 | ||
Molar mass | 286.32 g/mol | ||
Melting point | 205 to 211 °C (401 to 412 °F; 478 to 484 K)[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
1,1′-Bi-2-naphthol (BINOL) is an organic compound that is often used as a ligand for transition-metal catalysed asymmetric synthesis. BINOL has axial chirality and the two enantiomers can be readily separated and are stable toward racemisation. The specific rotation of the two enantiomers is ±35.5° (c = 1 in THF). BINOL is a precursor for another chiral ligand called BINAP.
Contents
1 Preparation
2 BINOL derivatives
3 See also
4 References
Preparation
The organic synthesis of BINOL is not a challenge as such but the preparation of the individual enantiomers is.
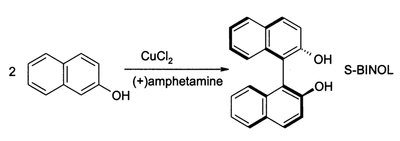
(S)-BINOL can be prepared directly from an asymmetric oxidative coupling of 2-naphthol with copper(II) chloride. The chiral ligand in this reaction is (S)-(+)-amphetamine.[2]
Racemic BINOL can also be produced using iron(III) chloride as an oxidant. The mechanism involves complexation of iron(III) into the hydroxyl, followed by a radical coupling reaction of the naphthol rings initiated by iron(III) reducing into iron(II).
Optically active BINOL can also be obtained from racemic BINOL by optical resolution. In one method, the alkaloid N-benzylcinchonidinium chloride forms a crystalline inclusion compound. The inclusion compound of the S-enantiomer is soluble in acetonitrile but that of the R-enantiomer is not.[3] In another method BINOL is esterified with pentanoyl chloride. The enzyme cholesterol esterase hydrolyses the (S)-diester but not the (R)-diester.[3] The (R)-dipentanoate is hydrolysed in a second step with sodium methoxide.[4] The third method employs HPLC with chiral stationary phases.[5]
BINOL derivatives
Aside from the starting materials derived directly from the chiral pool, (R)- and (S)-BINOL in high enantiopurity (>99% ee) are two of the most inexpensive sources of chirality for organic synthesis, costing less than $0.6/g when purchased in bulk from chemical suppliers.[6] As a consequence, it serves as an important starting material for other sources of chirality for stereoselective synthesis, both stoichiometric and substoichiometric (catalytic).
Many important chiral ligands are constructed from the binaphthyl scaffold and ultimately derived from BINOL as a starting material, BINAP being one of the most well known and important.
The compound aluminium lithium bis(binaphthoxide) (ALB) is prepared by reaction of BINOL with lithium aluminium hydride.[7] In a different stoichiometric ratio (1:1 BINOL/LiAlH4 instead of 2:1), the chiral reducing agent BINAL (lithium dihydrido(binaphthoxy)aluminate) is produced.[8]
It has been employed in an asymmetric Michael reaction with cyclohexenone and dimethyl malonate:
See also
Shibasaki catalysts
References
^ Datasheet, chemexper.com
^ Brussee, J.; Jansen, A. C. A. (1983). "A highly stereoselective synthesis of S-(−)-[1,1′-binaphthalene]-2,2′-diol". Tetrahedron Letters. 24 (31): 3261–3262. doi:10.1016/S0040-4039(00)88151-4..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ ab "RESOLUTION OF 1,1'-BI-2-NAPHTHOL", Dongwei Cai, David L. Hughes, Thomas R. Verhoeven, and Paul J. Reider, in Organic Syntheses Coll. Vol. 10, p.93; Vol. 76, p.1
^ "(S)-(−)- AND (R)-(+)-1,1′-BI-2-NAPHTHOL", Romas J. Kazlauskas in Organic Syntheses, Coll. Vol. 9, p.77; Vol. 70, p.60
^ Landek, G.; Vinković, M.; Kontrec, D.; Vinković, V. (2006). "Influence of mobile phase and temperature on separation of 1,1′-binaphthyl-2,2 '-diol enantiomers with brush type chiral stationary phases derived from L-leucine". Chromatographia. 64 (7–8): 469–473. doi:10.1365/s10337-006-0041-5.
^ Yang, Jin-Fei; Wang, Rong-Hua; Wang, Yin-Xia; Yao, Wei-Wei; Liu, Qi-Sheng; Ye, Mengchun (2016-10-11). "Ligand-Accelerated Direct C−H Arylation of BINOL: A Rapid One-Step Synthesis of Racemic 3,3′-Diaryl BINOLs". Angewandte Chemie International Edition. 55 (45): 14116–14120. doi:10.1002/anie.201607893. ISSN 1433-7851.
^ A practical large-scale synthesis of enantiomerically pure 3-[bis(methoxycarbonyl)methyl]cyclohexanone via catalytic asymmetric Michael reaction Tetrahedron, Volume 58, Issue 13, 25 March 2002, Pages 2585–2588 Youjun Xu, Ken Ohori, Takashi Ohshima, Masakatsu Shibasaki doi:10.1016/S0040-4020(02)00141-2
^ Gopalan, Aravamudan S.; Jacobs, Hollie K. (2001-04-15), "Lithium Aluminum Hydride-2,2′-Dihydroxy-1,1′-binaphthyl", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rl041, ISBN 0471936235, retrieved 2018-07-21





