Dihydrotestosterone
 | |
 | |
| Names | |
|---|---|
IUPAC name (5S,8R,9S,10S,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-one | |
| Other names DHT; 5α-Dihydrotestosterone; 5α-DHT; Androstanolone; Stanolone; 5α-Androstan-17β-ol-3-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChEMBL |
|
ChemSpider |
|
DrugBank |
|
ECHA InfoCard | 100.007.554 |
KEGG |
|
PubChem CID |
|
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C19H30O2 |
Molar mass | 290.45 g·mol−1 |
| Pharmacology | |
ATC code | A14AA01 (WHO) |
Routes of administration | Transdermal (gel), in the cheek, under the tongue, intramuscular injection (as esters) |
Pharmacokinetics: | |
Bioavailability | Oral: very low (due to extensive first pass metabolism)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Dihydrotestosterone (DHT, 5α-dihydrotestosterone, 5α-DHT, androstanolone or stanolone) is an endogenous androgen sex steroid and hormone. The enzyme 5α-reductase catalyzes the formation of DHT from testosterone in certain tissues including the prostate gland, seminal vesicles, epididymides, skin, hair follicles, liver, and brain. This enzyme mediates reduction of the C4-5 double bond of testosterone. Relative to testosterone, DHT is considerably more potent as an agonist of the androgen receptor (AR).
In addition to its role as a natural hormone, DHT has been used as a medication, for instance in the treatment of low testosterone levels in men; for information on DHT as a medication, see the androstanolone article.
Contents
1 Biological function
1.1 5α-Reductase deficiency
1.2 5α-Reductase inhibitors
2 Biological activity
3 Biochemistry
3.1 Biosynthesis
3.2 Distribution
3.3 Metabolism
3.4 Excretion
3.5 Levels
4 Medical use
5 Chemistry
6 History
7 References
Biological function
DHT is biologically important for sexual differentiation of the male genitalia during embryogenesis, maturation of the penis and scrotum at puberty, growth of facial, body, and pubic hair, and development and maintenance of the prostate gland and seminal vesicles. It is produced from the less potent testosterone by the enzyme 5α-reductase in select tissues, and is the primary androgen in the genitals, prostate gland, seminal vesicles, skin, and hair follicles.[2]
DHT signals mainly in an intracrine and paracrine manner in the tissues in which it is produced, playing only a minor role, if any, as a circulating endocrine hormone.[3][4][5] Circulating levels of DHT are 1/10th and 1/20th those of testosterone in terms of total and free concentrations, respectively,[6] whereas local DHT levels may be up to 10 times those of testosterone in tissues with high 5α-reductase expression such as the prostate gland.[7] In addition, unlike testosterone, DHT is inactivated by 3α-hydroxysteroid dehydrogenase (3α-HSD) into the very weak androgen 3α-androstanediol in various tissues such as muscle, adipose, and liver among others,[5][8][9] and in relation to this, DHT has been reported to be a very poor anabolic agent when administered exogenously as a medication.[10]
| Testosterone | DHT |
|---|---|
| Spermatogenesis and fertility | Prostate enlargement and prostate cancer risk |
| Male musculoskeletal development | Facial, axillary, pubic, and body hair growth |
| Voice deepening | Scalp temporal recession and pattern hair loss |
| Increased sebum production and acne | |
| Increased sex drive and erections |
In addition to normal biological functions, DHT also plays an important causative role in a number of androgen-dependent conditions including hair conditions like hirsutism (excessive facial/body hair growth) and pattern hair loss (androgenic alopecia or pattern baldness) and prostate diseases such as benign prostatic hyperplasia (BPH) and prostate cancer.[2]5α-Reductase inhibitors, which prevent DHT synthesis, are effective in the prevention and treatment of these conditions.[13][14][15][16]
Metabolites of DHT have been found to act as neurosteroids with their own AR-independent biological activity.[17]3α-Androstanediol is a potent positive allosteric modulator of the GABAA receptor, while 3β-androstanediol is a potent and selective agonist of the estrogen receptor (ER) subtype ERβ.[17] These metabolites may play important roles in the central effects of DHT and by extension testosterone, including their antidepressant, anxiolytic, rewarding/hedonic, anti-stress, and pro-cognitive effects.[17][18]
5α-Reductase deficiency
Much of the biological role of DHT has been elucidated in studies of individuals with congenital 5α-reductase type II deficiency, an intersex condition caused by a loss-of-function mutation in the gene encoding 5α-reductase type II, the major enzyme responsible for the production of DHT in the body.[13][19][2] It is characterized by a defective and non-functional 5α-reductase type II enzyme and a partial but majority loss of DHT production in the body.[13][19] In the condition, circulating testosterone levels are within or slightly above the normal male range, but DHT levels are low (around 30% of normal),[20][better source needed] and the ratio of circulating testosterone to DHT is greatly elevated (at about 3.5 to 5 times higher than normal).[13]
Genetic males (46,XY) with 5α-reductase type II deficiency are born with undervirilization including pseudohermaphroditism (ambiguous genitalia), pseudovaginal perineoscrotal hypospadias, and usually undescended testes. Their external genitalia are female-like, with micropenis (a small, clitoris-like phallus), a partially unfused, labia-like scrotum, and a blind-ending, shallow vaginal pouch.[13] Due to their lack of conspicuous male genitalia, genetic males with the condition are typically raised as girls.[19] At the time of puberty however, they develop striking phenotypically masculine secondary sexual characteristics including partial virilization of the genitals (enlargement of the phallus into a near-functional penis and descent of the testes), voice deepening, typical male musculoskeletal development,[12] and no menstruation, breast development, or other signs of feminization that occur during female puberty.[13][19][2] In addition, normal libido and spontaneous erections develop,[21] they usually show a sexual preference for females, and almost all develop a male gender identity.[13][22]
Nonetheless, males with 5α-reductase type II deficiency exhibit signs of continued undervirilization in a number of domains. Facial hair was absent or sparse in a relatively large group of Dominican males with the condition. However, more facial hair has been observed in patients with the disorder from other parts of the world, although facial hair was still reduced relative to that of other men in the same communities. The divergent findings may reflect racial differences in androgen-dependent hair growth. A female pattern of androgenic hair growth, with terminal hair largely restricted to the axillae and lower pubic triangle, is observed in males with the condition. No temporal recession of the hairline or androgenic alopecia (pattern hair loss or baldness) has been observed in any of the cases of 5α-reductase type II deficiency that have been reported, whereas this is normally seen to some degree in almost all Caucasian males.[13] Individuals with 5α-reductase type II deficiency were initially reported to have no incidence of acne,[8][2] but subsequent research indicated normal sebum secretion and acne incidence.[12]
In genetic males with 5α-reductase type II deficiency, the prostate gland is rudimentary or absent, and if present, remains small, underdeveloped, and unpalpable throughout life.[8][4] In addition, neither BPH nor prostate cancer have been reported in these individuals.[14] Genetic males with the condition generally show oligozoospermia due to undescended testes, but spermatogenesis is reported to be normal in those with testes that have descended, and there are case instances of men with the condition successfully fathering children.[21][23]
Unlike males, genetic females with 5α-reductase type II deficiency are phenotypically normal. However, similarly to genetic males with the condition, they show reduced body hair growth, including an absence of hair on the arms and legs, slightly decreased axillary hair, and moderately decreased pubic hair.[24][21] On the other hand, sebum production is normal.[24][25] This is in accordance with the fact that sebum secretion appears to be entirely under the control of 5α-reductase type I.[25]
5α-Reductase inhibitors
5α-Reductase inhibitors like finasteride and dutasteride inhibit 5α-reductase type II and/or other isoforms and are able to decrease circulating DHT levels by 65 to 98% depending on the 5α-reductase inhibitor in question.[26][27][28][20] As such, similarly to the case of 5α-reductase type II deficiency, they provide useful insights in the elucidation of the biological functions of DHT.[29] 5α-Reductase inhibitors were developed and are used primarily for the treatment of BPH. The drugs are able to significantly reduce the size of the prostate gland and to alleviate symptoms of the condition.[14][30] Long-term treatment with 5α-reductase inhibitors is also able to significantly reduce the overall risk of prostate cancer, although a simultaneous small increase in the risk of certain high-grade tumors has been observed.[15] In addition to prostate diseases, 5α-reductase inhibitors have subsequently been developed and introduced for the treatment of pattern hair loss in men.[31] They are able to prevent further progression of hair loss in most men with the condition and to produce some recovery of hair in about two-thirds of men.[13] 5α-Reductase inhibitors seem to be less effective for pattern hair loss in women on the other hand, although they do still show some effectiveness.[32] Aside from pattern hair loss, the drugs are also useful in the treatment of hirsutism and can greatly reduce facial and body hair growth in women with the condition.[33][16]
5α-Reductase inhibitors are overall well-tolerated and show a low incidence of adverse effects.[34]Sexual dysfunction, including erectile dysfunction, loss of libido, and reduced ejaculate volume, may occur in 3.4 to 15.8% of men treated with finasteride or dutasteride.[34][35] A small increase in the risk of affective symptoms including depression, anxiety, and self-harm may be seen.[36][37][38] Both the sexual dysfunction and affective symptoms may be due partially or fully to prevention of the synthesis of neurosteroids like allopregnanolone rather necessarily than due to inhibition of DHT production.[36] A very small risk of gynecomastia has been associated with 5α-reductase inhibitors (1.2 to 3.5%).[34][39] Based on reports of 5α-reductase type II deficiency in males and the effectiveness of 5α-reductase inhibitors for hirsutism in women, reduced body and/or facial hair growth is a likely potential side effect of these drugs in men.[13][16] There are very few studies evaluating the side effects of 5α-reductase inhibitors in women. However, due to the known role of DHT in male sexual differentiation, 5α-reductase inhibitors may cause birth defects such as ambiguous genitalia in the male fetuses of pregnant women. As such, they are not used in women during pregnancy.[34]
MK-386 is a selective 5α-reductase type I inhibitor which was never marketed.[40] Whereas 5α-reductase type II inhibitors achieve much higher reductions in circulating DHT production, MK-386 decreases circulating DHT levels by 20 to 30%.[41] Conversely, it was found to decrease sebum DHT levels by 55% in men versus a modest reduction of only 15% for finasteride.[42][43] However, MK-386 failed to show significant effectiveness in a subsequent clinical study for the treatment of acne.[44]
Biological activity
DHT is a potent agonist of the AR, and is in fact the most potent known endogenous ligand of the receptor. It has an affinity (Kd) of 0.25 to 0.5 nM for the human AR, which is about 2- to 3-fold higher than that of testosterone (Kd = 0.4 to 1.0 nM)[45] and 15–30 times higher than that of adrenal androgens.[46] In addition, the dissociation rate of DHT from the AR is 5-fold slower than that of testosterone.[47] The EC50 of DHT for activation of the AR is 0.13 nM, which is about 5-fold stronger than that of testosterone (EC50 = 0.66 nM).[48] In bioassays, DHT has been found to be 2.5- to 10-fold more potent than testosterone.[45]
The elimination half-life of DHT in the body (53 minutes) is longer than that of testosterone (34 minutes), and this may account for some of the difference in their potency.[49] A study of transdermal DHT and testosterone treatment reported terminal half-lives of 2.83 hours and 1.29 hours, respectively.[50]
Unlike other androgens such as testosterone, DHT cannot be converted by the enzyme aromatase into an estrogen like estradiol. Therefore, it is frequently used in research settings to distinguish between the effects of testosterone caused by binding to the AR and those caused by testosterone's conversion to estradiol and subsequent binding to and activation of ERs.[51] Although DHT cannot be aromatized, it is still transformed into metabolites with significant ER affinity and activity. These are 3α-androstanediol and 3β-androstanediol, which are predominant agonists of the ERβ.[17]
Biochemistry
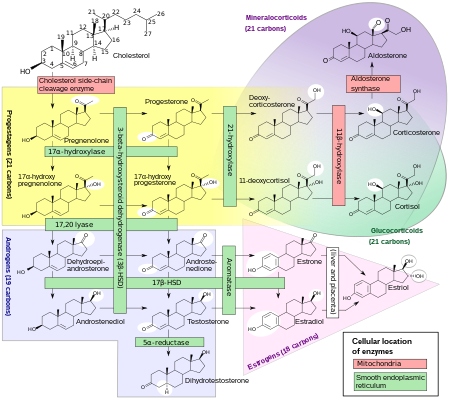
Comprehensive overview of steroidogenesis, showing DHT around the bottom middle among the androgens.[52]
Biosynthesis
DHT is synthesized irreversibly from testosterone by the enzyme 5α-reductase.[8][13] This occurs in various tissues including the genitals (penis, scrotum, clitoris, labia majora),[53]prostate gland, skin, hair follicles, liver, and brain.[8] Around 5 to 7% of testosterone undergoes 5α-reduction into DHT,[54][55] and approximately 200 to 300 μg of DHT is synthesized in the body per day. Most DHT is produced in peripheral tissues like the skin and liver, whereas most circulating DHT originates specifically from the liver. The testes and prostate gland contribute relatively little to concentrations of DHT in circulation.[8]
There are two major isoforms of 5α-reductase, SRD5A1 (type I) and SRD5A2 (type II), with the latter being the most biologically important isoenzyme.[8] There is also third 5α-reductase: SRD5A3.[56] SRD5A2 is most highly expressed in the genitals, prostate gland, epididymides, seminal vesicles, genital skin, facial and chest hair follicles,[57][58] and liver, while lower expression is observed in certain brain areas, non-genital skin/hair follicles, testes, and kidneys. SRD5A1 is most highly expressed in non-genital skin/hair follicles, the liver, and certain brain areas, while lower levels are present in the prostate, epididymides, seminal vesicles, genital skin, testes, adrenal glands, and kidneys.[8] In the skin, 5α-reductase is expressed in sebaceous glands, sweat glands, epidermal cells, and hair follicles.[57][58] Both isoenzymes are expressed in scalp hair follicles,[59] although SRD5A2 predominates in these cells.[58] The SRD5A2 subtype is the almost exclusive isoform expressed in the prostate gland.[60][20]
Distribution
The plasma protein binding of DHT is more than 99%. In men, approximately 0.88% of DHT is unbound and hence free, while in premenopausal women, about 0.47–0.48% is unbound. In men, DHT is bound 49.7% to sex hormone-binding globulin (SHBG), 39.2% to albumin, and 0.22% to corticosteroid-binding globulin (CBG), while in premenopausal women, DHT is bound 78.1–78.4% to SHBG, 21.0–21.3% to albumin, and 0.12% to CBG. In late pregnancy, only 0.07% of DHT is unbound in women; 97.8% is bound to SHBG while 2.15% is bound to albumin and 0.04% is bound to CBG.[61][62] DHT has higher affinity for SHBG than does testosterone, estradiol, or any other steroid hormone.[63][62]
| Compound | Group | Level (nM) | Free (%) | SHBG (%) | CBG (%) | Albumin (%) |
|---|---|---|---|---|---|---|
| Testosterone | Adult men | 23.0 | 2.23 | 44.3 | 3.56 | 49.9 |
| Adult women | ||||||
| Follicular phase | 1.3 | 1.36 | 66.0 | 2.26 | 30.4 | |
| Luteal phase | 1.3 | 1.37 | 65.7 | 2.20 | 30.7 | |
| Pregnancy | 4.7 | 0.23 | 95.4 | 0.82 | 3.6 | |
| Dihydrotestosterone | Adult men | 1.70 | 0.88 | 49.7 | 0.22 | 39.2 |
| Adult women | ||||||
| Follicular phase | 0.65 | 0.47 | 78.4 | 0.12 | 21.0 | |
| Luteal phase | 0.65 | 0.48 | 78.1 | 0.12 | 21.3 | |
| Pregnancy | 0.93 | 0.07 | 97.8 | 0.04 | 21.2 | |
Metabolism
DHT is inactivated in the liver and extrahepatic tissues like the skin into 3α-androstanediol and 3β-androstanediol by the enzymes 3α-hydroxysteroid dehydrogenase and 3β-hydroxysteroid dehydrogenase, respectively.[8][64] These metabolites are in turn converted, respectively, into androsterone and epiandrosterone, then conjugated (via glucuronidation and/or sulfation), released into circulation, and excreted in urine.[8]
Unlike testosterone, DHT cannot be aromatized into an estrogen like estradiol, and for this reason, has no propensity for estrogenic effects.[65]
Excretion
DHT is excreted in the urine as metabolites, such as conjugates of 3α-androstanediol and androsterone.[66][8]
Levels
Serum DHT levels are about 10% of those of testosterone, but levels in the prostate gland are 5- to 10-fold higher than those of testosterone due to a more than 90% conversion of testosterone into DHT by locally expressed 5α-reductase.[7] For this reason, and in addition to the fact that DHT is much more potent as an AR agonist than is testosterone,[45] DHT is considered to be the major androgen of the prostate gland.[7]
Medical use
DHT is available in pharmaceutical formulations for medical use as an androgen or anabolic–androgenic steroid (AAS).[67] It is used mainly in the treatment of male hypogonadism.[68] When used as a medication, dihydrotestosterone is referred to as androstanolone (INN) or as stanolone (BAN),[67][69][70] and is sold under brand names such as Andractim among others.[67][69][70][68][71] The availability of pharmaceutical DHT is limited; it is not available in the United States or Canada,[72][73] but is available in certain European countries.[70][68] The available formulations of DHT include buccal or sublingual tablets, topical gels, and, as esters in oil, injectables like androstanolone propionate and androstanolone valerate.[67][68][71]
Chemistry
DHT, also known as 5α-androstan-17β-ol-3-one, is a naturally occurring androstane steroid with a ketone group at the C3 position and a hydroxyl group at the C17β position. It is the derivative of testosterone in which the double bond between the C4 and C5 positions has been reduced or hydrogenated.
History
DHT was first synthesized by Adolf Butenandt and his colleagues in 1935.[74][75] It was prepared via hydrogenation of testosterone,[75] which had been discovered earlier that year.[76] DHT was introduced for medical use as an AAS in 1953, and was noted to be more potent than testosterone but with reduced androgenicity.[77][78][79] It was not elucidated to be an endogenous substance until 1956, when it was shown to be formed from testosterone in rat liver homogenates.[75][80] In addition, the biological importance of DHT was not realized until the early 1960s, when it was found to be produced by 5α-reductase from circulating testosterone in target tissues like the prostate gland and seminal vesicles and was found to be more potent than testosterone in bioassays.[81][82][83][84] The biological functions of DHT in humans became much more clearly defined upon the discovery and characterization of 5α-reductase type II deficiency in 1974.[14] DHT was the last major sex hormone, the others being testosterone, estradiol, and progesterone, to be discovered, and is unique in that it is the only major sex hormone that functions principally as an intracrine and paracrine hormone rather than as an endocrine hormone.[85]
References
^ Coutts, S. B.; Kicman, A. T.; Hurst, D. T.; Cowan, D. A. (1997-11-01). "Intramuscular administration of 5α-dihydrotestosterone heptanoate: changes in urinary hormone profile". Clinical Chemistry. 43 (11): 2091–2098. ISSN 0009-9147. PMID 9365393..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcde Marks LS (2004). "5α-reductase: history and clinical importance". Rev Urol. 6 Suppl 9: S11–21. PMC 1472916. PMID 16985920.
^ Horton R (1992). "Dihydrotestosterone is a peripheral paracrine hormone". J. Androl. 13 (1): 23–7. PMID 1551803.
^ ab Wilson JD (1996). "Role of dihydrotestosterone in androgen action". Prostate Suppl. 6: 88–92. PMID 8630237.
^ ab Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA (2017). "Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels". Endocr. Rev. 38 (3): 220–254. doi:10.1210/er.2016-1067. PMID 28472278.
^ Bhasin S (13 February 1996). Pharmacology, Biology, and Clinical Applications of Androgens: Current Status and Future Prospects. John Wiley & Sons. pp. 72–. ISBN 978-0-471-13320-9.
^ abc Hay ID, Wass JA (26 January 2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 37–. ISBN 978-1-4443-0023-9.
^ abcdefghijk Melmed S (2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 621, 711. ISBN 978-0-323-29738-7.
^ Jin Y, Penning TM (2001). "Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism". Best Pract. Res. Clin. Endocrinol. Metab. 15 (1): 79–94. doi:10.1053/beem.2001.0120. PMID 11469812.
^ Llewellyn W (2009). Anabolics. Molecular Nutrition Llc. pp. 19, 163. ISBN 978-0967930473.
^ Chang C (31 October 2002). Androgens and Androgen Receptor: Mechanisms, Functions, and Clinical Applications. Springer Science & Business Media. pp. 451–. ISBN 978-1-4020-7188-1.
^ abc Marchetti PM, Barth JH (2013). "Clinical biochemistry of dihydrotestosterone". Ann. Clin. Biochem. 50 (Pt 2): 95–107. doi:10.1258/acb.2012.012159. PMID 23431485.
^ abcdefghijk Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 161–162. ISBN 978-3-540-46911-7.
^ abcd Azzouni F, Mohler J (2012). "Role of 5α-reductase inhibitors in benign prostatic diseases". Prostate Cancer Prostatic Dis. 15 (3): 222–30. doi:10.1038/pcan.2012.1. PMID 22333687.
^ ab Azzouni F, Mohler J (2012). "Role of 5α-reductase inhibitors in prostate cancer prevention and treatment". Urology. 79 (6): 1197–205. doi:10.1016/j.urology.2012.01.024. PMID 22446342.
^ abc Lotti F, Maggi M (28 April 2015). "Hormonal Treatment for Skin Androgen-Related Disorders". In Katsambas A, Lotti T, Dessinioti C, D'Erme AM. European Handbook of Dermatological Treatments. Springer. pp. 1451–1464. ISBN 978-3-662-45139-7.
^ abcd Kohtz AS, Frye CA (2012). "Dissociating behavioral, autonomic, and neuroendocrine effects of androgen steroids in animal models". Methods Mol. Biol. 829: 397–431. doi:10.1007/978-1-61779-458-2_26. PMID 22231829.
^ Brunton PJ (2016). "Neuroactive steroids and stress axis regulation: Pregnancy and beyond". J. Steroid Biochem. Mol. Biol. 160: 160–8. doi:10.1016/j.jsbmb.2015.08.003. PMID 26259885.
^ abcd Okeigwe I, Kuohung W (2014). "5-Alpha reductase deficiency: a 40-year retrospective review". Curr Opin Endocrinol Diabetes Obes. 21 (6): 483–7. doi:10.1097/MED.0000000000000116. PMID 25321150.
^ abc Heesakkers J, Chapple C, Ridder DD, Farag F (24 February 2016). Practical Functional Urology. Springer. pp. 280–. ISBN 978-3-319-25430-2.
^ abc Imperato-McGinley J, Zhu YS (2002). "Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency". Mol. Cell. Endocrinol. 198 (1–2): 51–9. doi:10.1016/s0303-7207(02)00368-4. PMID 12573814.
^ Imperato-McGinley J, Peterson RE, Gautier T, Sturla E (1979). "Androgens and the evolution of male-gender identity among male pseudohermaphrodites with 5alpha-reductase deficiency". N. Engl. J. Med. 300 (22): 1233–7. doi:10.1056/NEJM197905313002201. PMID 431680.
^ Kang HJ, Imperato-McGinley J, Zhu YS, Rosenwaks Z (2014). "The effect of 5α-reductase-2 deficiency on human fertility". Fertil. Steril. 101 (2): 310–6. doi:10.1016/j.fertnstert.2013.11.128. PMC 4031759. PMID 24412121.
^ ab Katz MD, Cai LQ, Zhu YS, Herrera C, DeFillo-Ricart M, Shackleton CH, Imperato-McGinley J (1995). "The biochemical and phenotypic characterization of females homozygous for 5 alpha-reductase-2 deficiency". J. Clin. Endocrinol. Metab. 80 (11): 3160–7. doi:10.1210/jcem.80.11.7593420. PMID 7593420.
^ ab Cilotti A, Danza G, Serio M (2001). "Clinical application of 5alpha-reductase inhibitors". J. Endocrinol. Invest. 24 (3): 199–203. doi:10.1007/bf03343844. PMID 11314752.
^ Bradbury R (30 January 2007). Cancer. Springer Science & Business Media. pp. 49–. ISBN 978-3-540-33120-9.
^ Burchum J, Rosenthal L (2 December 2014). Lehne's Pharmacology for Nursing Care. Elsevier Health Sciences. pp. 803–. ISBN 978-0-323-34026-7.
^ Bostwick DG, Cheng L (24 January 2014). Urologic Surgical Pathology. Elsevier Health Sciences. pp. 492–. ISBN 978-0-323-08619-6.
^ Harris GS, Kozarich JW (1997). "Steroid 5alpha-reductase inhibitors in androgen-dependent disorders". Curr Opin Chem Biol. 1 (2): 254–9. doi:10.1016/s1367-5931(97)80017-8. PMID 9667860.
^ Sun J, Xiang H, Yang LL, Chen JB (2011). "A review on steroidal 5α-reductase inhibitors for treatment of benign prostatic hyperplasia". Curr. Med. Chem. 18 (23): 3576–89. doi:10.2174/092986711796642517. PMID 21756226.
^ Torres F (2015). "Androgenetic, diffuse and senescent alopecia in men: practical evaluation and management". Curr. Probl. Dermatol. 47: 33–44. doi:10.1159/000369403. PMID 26370642.
^ Check JH, Cohen R (2015). "An update on the treatment of female alopecia and the introduction of a potential novel therapy". Clin Exp Obstet Gynecol. 42 (4): 411–5. PMID 26411201.
^ Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 182, 369. ISBN 978-3-540-46911-7.
^ abcd Hirshburg JM, Kelsey PA, Therrien CA, Gavino AC, Reichenberg JS (2016). "Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review". J Clin Aesthet Dermatol. 9 (7): 56–62. PMC 5023004. PMID 27672412.
^ Liu L, Zhao S, Li F, Li E, Kang R, Luo L, Luo J, Wan S, Zhao Z (2016). "Effect of 5α-Reductase Inhibitors on Sexual Function: A Meta-Analysis and Systematic Review of Randomized Controlled Trials". J Sex Med. 13 (9): 1297–310. doi:10.1016/j.jsxm.2016.07.006. PMID 27475241.
^ ab Traish AM, Mulgaonkar A, Giordano N (2014). "The dark side of 5α-reductase inhibitors' therapy: sexual dysfunction, high Gleason grade prostate cancer and depression". Korean J Urol. 55 (6): 367–79. doi:10.4111/kju.2014.55.6.367. PMC 4064044. PMID 24955220.
^ Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S (2017). "Association of Suicidality and Depression With 5α-Reductase Inhibitors". JAMA Intern Med. 177 (5): 683–691. doi:10.1001/jamainternmed.2017.0089. PMID 28319231.
^ Thielke S (2017). "The Risk of Suicidality and Depression From 5-α Reductase Inhibitors". JAMA Intern Med. 177 (5): 691–692. doi:10.1001/jamainternmed.2017.0096. PMID 28319227.
^ Fertig R, Shapiro J, Bergfeld W, Tosti A (2017). "Investigation of the Plausibility of 5-Alpha-Reductase Inhibitor Syndrome". Skin Appendage Disord. 2 (3–4): 120–129. doi:10.1159/000450617. PMC 5264352. PMID 28232919.
^ Machetti F, Guarna A (2005). "Novel inhibitors of 5α-reductase". Expert Opinion on Therapeutic Patents. 12 (2): 201–215. doi:10.1517/13543776.12.2.201. ISSN 1354-3776.
^ Schwartz JI, Van Hecken A, De Schepper PJ, De Lepeleire I, Lasseter KC, Shamblen EC, Winchell GA, Constanzer ML, Chavez CM, Wang DZ, Ebel DL, Justice SJ, Gertz BJ (1996). "Effect of MK-386, a novel inhibitor of type 1 5 alpha-reductase, alone and in combination with finasteride, on serum dihydrotestosterone concentrations in men". J. Clin. Endocrinol. Metab. 81 (8): 2942–7. doi:10.1210/jcem.81.8.8768856. PMID 8768856.
^ Schwartz JI, Tanaka WK, Wang DZ, Ebel DL, Geissler LA, Dallob A, Hafkin B, Gertz BJ (1997). "MK-386, an inhibitor of 5alpha-reductase type 1, reduces dihydrotestosterone concentrations in serum and sebum without affecting dihydrotestosterone concentrations in semen". J. Clin. Endocrinol. Metab. 82 (5): 1373–7. doi:10.1210/jcem.82.5.3912. PMID 9141518.
^ Kaufman, Keith D. (2001). "5α-Reductase Inhibitors in the Treatment of Androgenetic Alopecia". International Journal of Cosmetic Surgery and Aesthetic Dermatology. 3 (2): 107–119. doi:10.1089/153082001753231036. ISSN 1530-8200.
^ Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Adv Urol. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
^ abc Mozayani A, Raymon L (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
^ Hemat RA (2004). Principles Of Orthomolecularism. Urotext. p. 426. ISBN 1-903737-05-2.
^ Grino PB, Griffin JE, Wilson JD (February 1990). "Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone". Endocrinology. 126 (2): 1165–72. doi:10.1210/endo-126-2-1165. PMID 2298157.
^ Wilderer PA (1 September 2010). "Bioassays for Estrogenic and Androgenic Effects of Water Constituents". Treatise on Water Science, Four-Volume Set. Newnes. pp. 1805–. ISBN 978-0-444-53199-5.
^ Diamanti-Kandarakis E (1999). "Current aspects of antiandrogen therapy in women". Current Pharmaceutical Design. 5 (9): 707–23. PMID 10495361.
^ von Deutsch DA, Abukhalaf IK, Lapu-Bula R (15 October 2003). "Anabolic Doping Agents". In Mozayani A, Raymon L. Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 510–. doi:10.1007/978-1-61779-222-9_15. ISBN 978-1-59259-654-6.
^ Swerdloff RS, Wang C (October 1998). "Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent". Baillière's Clinical Endocrinology and Metabolism. 12 (3): 501–6. doi:10.1016/s0950-351x(98)80267-x. PMID 10332569.
^ Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
^ Rhoades RA, Bell DR (18 January 2012). Medical Phisiology: Principles for Clinical Medicine. Lippincott Williams & Wilkins. pp. 690–. ISBN 978-1-60913-427-3.
^ Rakel D (12 April 2012). Integrative Medicine E-Book. Elsevier Health Sciences. pp. 321–. ISBN 1-4557-2503-X.
^ Morrison MF (4 May 2000). Hormones, Gender and the Aging Brain: The Endocrine Basis of Geriatric Psychiatry. Cambridge University Press. pp. 17–. ISBN 978-1-139-42645-9.
^ Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Advances in Urology. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
^ ab Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R (2007). "Sexual hormones in human skin". Horm. Metab. Res. 39 (2): 85–95. doi:10.1055/s-2007-961807. PMID 17326004.
^ abc Bolognia JL, Jorizzo JL, Schaffer JV (8 June 2012). Dermatology E-Book. Elsevier Health Sciences. pp. 1094–. ISBN 0-7020-5182-9.
^ Murphy MJ (24 March 2011). Molecular Diagnostics in Dermatology and Dermatopathology. Springer Science & Business Media. pp. 373–. ISBN 978-1-60761-171-4.
^ Keam SJ, Scott LJ (2008). "Dutasteride: a review of its use in the management of prostate disorders". Drugs. 68 (4): 463–85. doi:10.2165/00003495-200868040-00008. PMID 18318566.
^ ab Nieschlag E, Behre HM, Nieschlag S (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 61–. ISBN 978-1-107-01290-5.
^ ab Dunn JF, Nisula BC, Rodbard D (July 1981). "Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 58–68. doi:10.1210/jcem-53-1-58. PMID 7195404.
^ Williams DA, Foye WO, Lemke TL (2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 707–. ISBN 978-0-683-30737-5.
^ Rizner TL, Lin HK, Peehl DM, Steckelbroeck S, Bauman DR, Penning TM (July 2003). "Human type 3 3alpha-hydroxysteroid dehydrogenase (aldo-keto reductase 1C2) and androgen metabolism in prostate cells". Endocrinology. 144 (7): 2922–32. doi:10.1210/en.2002-0032. PMID 12810547.
^ Weiner IB, Gallagher M (2003). Handbook of Psychology, Biological Psychology. John Wiley & Sons. pp. 333–. ISBN 978-0-471-38403-8.
^ Schill W, Comhaire FH, Hargreave TB (26 August 2006). Andrology for the Clinician. Springer Science & Business Media. pp. 243–. ISBN 978-3-540-33713-3.
^ abcd Hyde TE, Gengenbach MS (2007). Conservative Management of Sports Injuries. Jones & Bartlett Learning. pp. 1100–. ISBN 978-0-7637-3252-3.
^ abcd "Androstanolone Drug Profile". Adis Insight. 4 December 2006.
^ ab Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 640–. ISBN 978-1-4757-2085-3.
^ abc Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 63–. ISBN 978-3-88763-075-1.
^ ab List PH, Hörhammer L (12 March 2013). Chemikalien und Drogen: Teil B: R, S. Springer-Verlag. pp. 523–. ISBN 978-3-642-66377-2.
^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
^ "Drug Product Database - Health Canada". Health Canada. Retrieved 13 November 2016.
^ Schnitzer R (1 January 1967). Experimental Chemotherapy. Elsevier Science. pp. 156–. ISBN 978-0-323-14611-1.
^ abc Krüskemper H (22 October 2013). Anabolic Steroids. Elsevier. pp. 12–. ISBN 978-1-4832-6504-9.
^ Taylor WN (16 January 2002). Anabolic Steroids and the Athlete, 2d ed. McFarland. pp. 178–. ISBN 978-0-7864-1128-3.
^ William Andrew Publishing (2007). Pharmaceutical Manufacturing Encyclopedia. William Andrew Pub. ISBN 978-0-8155-1526-5.
^ Newsweek. Newsweek. 1953.
^ New and Nonofficial Drugs. Lippincott. 1958.
^ Rubin BL, Dorfman RI (1956). "In vitro conversion of testosterone to 17beta-hydroxyandrostan-3-one". Proc. Soc. Exp. Biol. Med. 91 (4): 585–6. doi:10.3181/00379727-91-22337. PMID 13323010.
^ Agmo A (18 April 2011). Functional and Dysfunctional Sexual Behavior: A Synthesis of Neuroscience and Comparative Psychology. Academic Press. pp. 196–. ISBN 978-0-08-054938-5.
^ Oreopoulos DG, Michelis M, Herschorn S (6 December 2012). Nephrology and Urology in the Aged Patient. Springer Science & Business Media. pp. 495–. ISBN 978-94-011-1822-4.
^ Webster GF, Rawlings AV (17 May 2007). Acne and Its Therapy. CRC Press. pp. 168–. ISBN 978-1-4200-1841-7.
^ Smith LB, Mitchell RT, McEwan IJ (1 October 2013). Testosterone: From Basic Research to Clinical Applications. Springer Science & Business Media. pp. 5–. ISBN 978-1-4614-8978-8.
^ Anawalt BD (2017). "Is Dihydrotestosterone a Classic Hormone?". Endocr. Rev. 38 (3): 170–172. doi:10.1210/er.2017-00091. PMID 28582536.