Graphite
| Graphite | |
|---|---|
 Graphite specimen | |
| General | |
| Category | Native mineral |
Formula .mw-parser-output .nobold{font-weight:normal} (repeating unit) | C |
| Strunz classification | 1.CB.05a |
| Crystal system | Hexagonal |
| Crystal class | Dihexagonal dipyramidal (6/mmm) Hermann–Mauguin notation: (6/m 2/m 2/m) |
| Space group | P63mc |
| Unit cell | a = 2.461, c = 6.708 [Å]; Z = 4 |
| Identification | |
| Color | Iron-black to steel-gray; deep blue in transmitted light |
| Crystal habit | Tabular, six-sided foliated masses, granular to compacted masses |
| Twinning | Present |
| Cleavage | Basal – perfect on {0001} |
| Fracture | Flaky, otherwise rough when not on cleavage |
| Tenacity | Flexible non-elastic, sectile |
Mohs scale hardness | 1–2 |
| Luster | Metallic, earthy |
| Streak | Black |
| Diaphaneity | Opaque, transparent only in extremely thin flakes |
| Specific gravity | 1.9–2.3 |
| Density | 2.09–2.23 g/cm3 |
| Optical properties | Uniaxial (–) |
| Pleochroism | Strong |
| Solubility | Soluble in molten nickel, warm chlorosulfuric acid[1] |
| Other characteristics | strongly anisotropic, conducts electricity, greasy feel, readily marks |
| References | [2][3][4] |
Graphite (/ˈɡræfaɪt/), archaically referred to as plumbago, is a crystalline allotrope of carbon, a semimetal, a native element mineral, and a form of coal.[5] Graphite is the most stable form of carbon under standard conditions. Therefore, it is used in thermochemistry as the standard state for defining the heat of formation of carbon compounds.
Contents
1 Types and varieties
2 Occurrence
3 Properties
3.1 Structure
3.2 Other properties
4 History of natural graphite use
4.1 Other names
5 Uses of natural graphite
5.1 Refractories
5.2 Batteries
5.3 Steelmaking
5.4 Brake linings
5.5 Foundry facings and lubricants
5.6 Pencils
5.7 Other uses
5.8 Expanded graphite
5.9 Intercalated graphite
6 Uses of synthetic graphite
6.1 Invention of a process to produce synthetic graphite
6.2 Scientific research
6.3 Electrodes
6.4 Powder and scrap
6.5 Neutron moderator
6.6 Other uses
7 Graphite mining, beneficiation, and milling
7.1 Occupational safety
7.1.1 United States
8 Graphite recycling
9 See also
10 References
11 Further reading
12 External links
Types and varieties
The principal types of natural graphite, each occurring in different types of ore deposits are:
Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular;- Amorphous graphite: very fine flake graphite is sometimes called amorphous;[6]
- Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline aggregates, and is probably hydrothermal in origin.[7]
Highly ordered pyrolytic graphite refers to graphite with an angular spread between the graphite sheets of less than 1°.[8]
- The name "graphite fiber" is sometimes used to refer to carbon fibers or carbon fiber-reinforced polymer.
Occurrence

Graphite output in 2005
Graphite occurs in metamorphic rocks as a result of the reduction of sedimentary carbon compounds during metamorphism. It also occurs in igneous rocks and in meteorites.[4] Minerals associated with graphite include quartz, calcite, micas and tourmaline. In meteorites it occurs with troilite and silicate minerals.[4] Small graphitic crystals in meteoritic iron are called cliftonite.[7]
According to the United States Geological Survey (USGS), world production of natural graphite in 2016 was 1,200,000 tonnes, of which the following major exporters are: China (780,000 t), India (170,000 t), Brazil (80,000 t), Turkey (32,000 t) and North Korea (30,000 t).[9] Graphite is not mined in the United States, but U.S. production of synthetic graphite in 2010 was 134,000 t valued at $1.07 billion.[10]
Properties
Structure
Graphite has a layered, planar structure. The individual layers are called graphene. In each layer, the carbon atoms are arranged in a honeycomb lattice with separation of 0.142 nm, and the distance between planes is 0.335 nm.[11] Atoms in the plane are bonded covalently, with only three of the four potential bonding sites satisfied. The fourth electron is free to migrate in the plane, making graphite electrically conductive. However, it does not conduct in a direction at right angles to the plane. Bonding between layers is via weak van der Waals bonds, which allows layers of graphite to be easily separated, or to slide past each other.
The two known forms of graphite, alpha (hexagonal) and beta (rhombohedral), have very similar physical properties, except for that the graphene layers stack slightly differently.[12] The alpha graphite may be either flat or buckled.[13] The alpha form can be converted to the beta form through mechanical treatment and the beta form reverts to the alpha form when it is heated above 1300 °C.[14]

Scanning tunneling microscope image of graphite surface atoms

Graphite's unit cell

Animated view of the unit cell in three layers of graphene (note that this is a slightly different unit cell from the one to the left)

Ball-and-stick model of graphite (two graphene layers)

Side view of layer stacking

Plane view of layer stacking

Rotating graphite stereogram
Other properties

Graphite plates and sheets, 10–15 cm high, Mineral specimen from Kimmirut, Baffin Island
The acoustic and thermal properties of graphite are highly anisotropic, since phonons propagate quickly along the tightly bound planes, but are slower to travel from one plane to another. Graphite's high thermal stability and electrical and thermal conductivity facilitate its widespread use as electrodes and refractories in high temperature material processing applications. However, in oxygen-containing atmospheres graphite readily oxidizes to form carbon dioxide at temperatures of 700 °C and above.[15]

Molar volume against pressure at room temperature
Graphite is an electrical conductor, hence useful in such applications as arc lamp electrodes. It can conduct electricity due to the vast electron delocalization within the carbon layers (a phenomenon called aromaticity). These valence electrons are free to move, so are able to conduct electricity. However, the electricity is primarily conducted within the plane of the layers. The conductive properties of powdered graphite[16] allow its use as pressure sensor in carbon microphones.
Graphite and graphite powder are valued in industrial applications for their self-lubricating and dry lubricating properties. There is a common belief that graphite's lubricating properties are solely due to the loose interlamellar coupling between sheets in the structure.[17] However, it has been shown that in a vacuum environment (such as in technologies for use in space), graphite degrades as a lubricant, due to the hypoxic conditions.[18] This observation led to the hypothesis that the lubrication is due to the presence of fluids between the layers, such as air and water, which are naturally adsorbed from the environment. This hypothesis has been refuted by studies showing that air and water are not absorbed.[19] Recent studies suggest that an effect called superlubricity can also account for graphite's lubricating properties. The use of graphite is limited by its tendency to facilitate pitting corrosion in some stainless steel,[20][21] and to promote galvanic corrosion between dissimilar metals (due to its electrical conductivity). It is also corrosive to aluminium in the presence of moisture. For this reason, the US Air Force banned its use as a lubricant in aluminium aircraft,[22] and discouraged its use in aluminium-containing automatic weapons.[23] Even graphite pencil marks on aluminium parts may facilitate corrosion.[24] Another high-temperature lubricant, hexagonal boron nitride, has the same molecular structure as graphite. It is sometimes called white graphite, due to its similar properties.
When a large number of crystallographic defects bind these planes together, graphite loses its lubrication properties and becomes what is known as pyrolytic graphite. It is also highly anisotropic, and diamagnetic, thus it will float in mid-air above a strong magnet. If it is made in a fluidized bed at 1000–1300 °C then it is isotropic turbostratic, and is used in blood contacting devices like mechanical heart valves and is called pyrolytic carbon, and is not diamagnetic. Pyrolytic graphite and pyrolytic carbon are often confused but are very different materials.[citation needed]
Natural and crystalline graphites are not often used in pure form as structural materials, due to their shear-planes, brittleness, and inconsistent mechanical properties.
History of natural graphite use
In the 4th millennium BC, during the Neolithic Age in southeastern Europe, the Marița culture used graphite in a ceramic paint for decorating pottery.[25]
Some time before 1565 (some sources say as early as 1500), an enormous deposit of graphite was discovered on the approach to Grey Knotts from the hamlet of Seathwaite in Borrowdale parish, Cumbria, England, which the locals found useful for marking sheep.[26][27] During the reign of Elizabeth I (1558–1603), Borrowdale graphite was used as a refractory material to line moulds for cannonballs, resulting in rounder, smoother balls that could be fired farther, contributing to the strength of the English navy. This particular deposit of graphite was extremely pure and soft, and could easily be cut into sticks. Because of its military importance, this unique mine and its production were strictly controlled by the Crown.[28]
During the 19th century, graphite's uses greatly expanded to include stove polish, lubricants, paints, crucibles, foundry facings, and pencils, a major factor in the expansion of educational tools during the first great rise of education for the masses. The British empire controlled most of the world's production (especially from Ceylon), but production from Austrian, German and American deposits expanded by mid-century. For example, the Dixon Crucible Company of Jersey City, New Jersey, founded by Joseph Dixon (inventor) and partner Orestes Cleveland in 1845, opened mines in the Lake Ticonderoga district of New York, built a processing plant there, and a factory to manufacture pencils, crucibles and other products in New Jersey, described in the Engineering & Mining Journal 21 December 1878. The Dixon pencil is still in production.[29]
The beginnings of the revolutionary froth flotation process are associated with graphite mining. Included in the E&MJ article on the Dixon Crucible Company is a sketch of the “floating tanks” used in the age-old process of extracting graphite. Because graphite is so light, the mix of graphite and waste was sent through a final series of water tanks where a cleaner graphite “floated” off, which left waste to drop out. In an 1877 patent, the two brothers Bessel (Adolph and August) of Dresden, Germany took this “floating” process a step further and added a small amount of oil to the tanks and boiled the mix – an agitation or frothing step – to collect the graphite, the first steps toward the future flotation process. Adolph Bessel received the Wohler Medal for the patented process that upgraded the recovery of graphite to 90% from the German deposit. In 1977, the German Society of Mining Engineers and Metallurgists organized a special symposium dedicated to their discovery and, thus, the 100th anniversary of flotation.[30]
In the United States, in 1885, Hezekiah Bradford of Philadelphia, patented a similar process, but it is uncertain if his process was used successfully in the nearby graphite deposits of Chester County, Pennsylvania, a major producer by the 1890s. The Bessel process was limited in use, primarily because of the abundant cleaner deposits found around the globe, which needed not much more than hand-sorting to gather the pure graphite. The state of the art, ca. 1900, is described in the Canadian Department of Mines report on graphite mines and mining, when Canadian deposits began to become important producers of graphite.[31][32]
Other names
Historically, graphite was called black lead or plumbago.[7][33] Plumbago was commonly used in its massive mineral form. Both of these names arise from confusion with the similar-appearing lead ores, particularly galena. The Latin word for lead, plumbum, gave its name to the English term for this grey metallic-sheened mineral and even to the leadworts or plumbagos, plants with flowers that resemble this colour.
The term black lead usually refers to a powdered or processed graphite, matte black in color.
Abraham Gottlob Werner coined the name graphite ("writing stone") in 1789. He attempted to clear up the confusion between molybdena, plumbago and black lead after Carl Wilhelm Scheele in 1778 proved that there are at least three different minerals. Scheele's analysis showed that the chemical compounds molybdenum sulfide (molybdenite), lead(II) sulfide (galena) and graphite were three different soft black minerals.[34][35][36]
Uses of natural graphite
Natural graphite is mostly consumed for refractories, batteries, steelmaking, expanded graphite, brake linings, foundry facings and lubricants.[10]Graphene, which occurs naturally in graphite, has unique physical properties and is among the strongest substances known. However, the process of separating it from graphite will require more technological development.
Refractories
The use of graphite as a refractory material began before 1900 with the graphite crucible used to hold molten metal; this is now a minor part of refractories. In the mid-1980s, the carbon-magnesite brick became important, and a bit later the alumina-graphite shape. As of 2017[update] the order of importance is: alumina-graphite shapes, carbon-magnesite brick, monolithics (gunning and ramming mixes), and then crucibles.
Crucibles began using very large flake graphite, and carbon-magnesite brick requiring not quite so large flake graphite; for these and others there is now much more flexibility in the size of flake required, and amorphous graphite is no longer restricted to low-end refractories. Alumina-graphite shapes are used as continuous casting ware, such as nozzles and troughs, to convey the molten steel from ladle to mold, and carbon magnesite bricks line steel converters and electric-arc furnaces to withstand extreme temperatures. Graphite blocks are also used in parts of blast furnace linings where the high thermal conductivity of the graphite is critical. High-purity monolithics are often used as a continuous furnace lining instead of carbon-magnesite bricks.
The US and European refractories industry had a crisis in 2000–2003, with an indifferent market for steel and a declining refractory consumption per tonne of steel underlying firm buyouts and many plant closures.[citation needed] Many of the plant closures resulted from the acquisition of Harbison-Walker Refractories by RHI AG and some plants had their equipment auctioned off. Since much of the lost capacity was for carbon-magnesite brick, graphite consumption within the refractories area moved towards alumina-graphite shapes and monolithics, and away from brick. The major source of carbon-magnesite brick is now imports from China. Almost all of the above refractories are used to make steel and account for 75% of refractory consumption; the rest is used by a variety of industries, such as cement.
According to the USGS, US natural graphite consumption in refractories comprised 12,500 tonnes in 2010.[10]
Batteries
The use of graphite in batteries has increased in the last 30 years. Natural and synthetic graphite are used to construct electrodes in major battery technologies.[10][not in citation given] The lithium-ion battery utilizes roughly twice the amount of graphite as lithium carbonate.[37]
The demand for batteries, primarily nickel–metal hydride and lithium-ion batteries, caused a growth in demand for graphite in the late 1980s and early 1990s - a growth driven by portable electronics, such as portable CD players and power tools. Laptops, mobile phones, tablets, and smartphone products have increased the demand for batteries. Electric-vehicle batteries are anticipated[by whom?] to increase graphite demand. As an example, a lithium-ion battery in a fully electric Nissan Leaf contains nearly 40 kg of graphite.
Steelmaking
Natural graphite in steelmaking mostly goes into raising the carbon content in molten steel, and can also be used to lubricate the dies used to extrude hot steel. Carbon additives are subject to competitive pricing from alternatives such as synthetic graphite powder, petroleum coke, and other forms of carbon. A carbon raiser is added to increase the carbon content of the steel to the specified level. An estimate based on USGS's graphite consumption statistics indicates that 10,500 tonnes were used in this fashion in the US in 2005.[10]
Brake linings
Natural amorphous and fine flake graphite are used in brake linings or brake shoes for heavier (nonautomotive) vehicles, and became important with the need to substitute for asbestos. This use has been important for quite some time, but nonasbestos organic (NAO) compositions are beginning to reduce graphite's market share. A brake-lining industry shake-out with some plant closures has not been beneficial, nor has an indifferent automotive market. According to the USGS, US natural graphite consumption in brake linings was 6,510 tonnes in 2005.[10]
Foundry facings and lubricants
A foundry facing mold wash is a water-based paint of amorphous or fine flake graphite. Painting the inside of a mold with it and letting it dry leaves a fine graphite coat that will ease separation of the object cast after the hot metal has cooled. Graphite lubricants are specialty items for use at very high or very low temperatures, as forging die lubricant, an antiseize agent, a gear lubricant for mining machinery, and to lubricate locks. Having low-grit graphite, or even better, no-grit graphite (ultra high purity), is highly desirable. It can be used as a dry powder, in water or oil, or as colloidal graphite (a permanent suspension in a liquid). An estimate based on USGS graphite consumption statistics indicates that 2,200 tonnes was used in this fashion in 2005.[10]
Pencils

Graphite pencils
The ability to leave marks on paper and other objects gave graphite its name, given in 1789 by German mineralogist Abraham Gottlob Werner. It stems from graphein, meaning to write or draw in Ancient Greek.[7][38]
From the 16th century, all pencils were made with leads of English natural graphite, but modern pencil lead is most commonly a mix of powdered graphite and clay; it was invented by Nicolas-Jacques Conté in 1795.[39][40] It is chemically unrelated to the metal lead, whose ores had a similar appearance, hence the continuation of the name. Plumbago is another older term for natural graphite used for drawing, typically as a lump of the mineral without a wood casing. The term plumbago drawing is normally restricted to 17th and 18th century works, mostly portraits.
Today, pencils are still a small but significant market for natural graphite. Around 7% of the 1.1 million tonnes produced in 2011 was used to make pencils.[37] Low-quality amorphous graphite is used and sourced mainly from China.[10]
Other uses
Natural graphite has found uses in zinc-carbon batteries, in electric motor brushes, and various specialized applications. Graphite of various hardness or softness results in different qualities and tones when used as an artistic medium.[41] Railroads would often mix powdered graphite with waste oil or linseed oil to create a heat-resistant protective coating for the exposed portions of a steam locomotive's boiler, such as the smokebox or lower part of the firebox.[42]
Expanded graphite
Expanded graphite is made by immersing natural flake graphite in a bath of chromic acid, then concentrated sulfuric acid, which forces the crystal lattice planes apart, thus expanding the graphite. The expanded graphite can be used to make graphite foil or used directly as "hot top" compound to insulate molten metal in a ladle or red-hot steel ingots and decrease heat loss, or as firestops fitted around a fire door or in sheet metal collars surrounding plastic pipe (during a fire, the graphite expands and chars to resist fire penetration and spread), or to make high-performance gasket material for high-temperature use. After being made into graphite foil, the foil is machined and assembled into the bipolar plates in fuel cells.
The foil is made into heat sinks for laptop computers which keeps them cool while saving weight, and is made into a foil laminate that can be used in valve packings or made into gaskets. Old-style packings are now a minor member of this grouping: fine flake graphite in oils or greases for uses requiring heat resistance. A GAN estimate of current US natural graphite consumption in this end use is 7,500 tonnes.[10]
Intercalated graphite
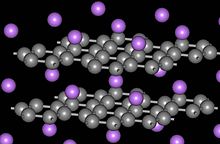
Structure of CaC6
Graphite forms intercalation compounds with some metals and small molecules. In these compounds, the host molecule or atom gets "sandwiched" between the graphite layers, resulting in a type of compound with variable stoichiometry. A prominent example of an intercalation compound is potassium graphite, denoted by the formula KC8. Some graphite intercalation compounds are superconductors. The highest transition temperature (by June 2009) Tc = 11.5 K is achieved in CaC6, and it further increases under applied pressure (15.1 K at 8 GPa).[43] Graphite's ability to intercalate lithium ions without significant damage from swelling is what makes it the dominant anode material in lithium-ion batteries.
Uses of synthetic graphite
Invention of a process to produce synthetic graphite
In 1893, Charles Street of Le Carbone discovered a process for making artificial graphite. Another process to make synthetic graphite was invented accidentally by Edward Goodrich Acheson (1856–1931). In the mid-1890s, Acheson discovered that overheating carborundum (silicon carbide or SiC) produced almost pure graphite. While studying the effects of high temperature on carborundum, he had found that silicon vaporizes at about 4,150 °C (7,500 °F), leaving the carbon behind in graphitic carbon. This graphite was another major discovery for him, and it became extremely valuable and helpful as a lubricant.[7]
In 1896, Acheson received a patent for his method of synthesizing graphite,[44] and in 1897 started commercial production.[7] The Acheson Graphite Co. was formed in 1899.
Scientific research
Highly oriented pyrolytic graphite (HOPG) is the highest-quality synthetic form of graphite. It is used in scientific research, in particular, as a length standard for scanner calibration of scanning probe microscope.[45]
Electrodes
Graphite electrodes carry the electricity that melts scrap iron and steel, and sometimes direct-reduced iron (DRI), in electric arc furnaces, which are the vast majority of steel furnaces. They are made from petroleum coke after it is mixed with coal tar pitch. They are then extruded and shaped, baked to carbonize the binder (pitch), and finally graphitized by heating it to temperatures approaching 3000 °C, at which the carbon atoms arrange into graphite. They can vary in size up to 3.5 m (11 ft) long and 75 cm (30 in) in diameter. An increasing proportion of global steel is made using electric arc furnaces, and the electric arc furnace itself is becoming more efficient, making more steel per tonne of electrode. An estimate based on USGS data indicates that graphite electrode consumption was 197,000 tonnes in 2005.[10]
Electrolytic aluminium smelting also uses graphitic carbon electrodes. On a much smaller scale, synthetic graphite electrodes are used in electrical discharge machining (EDM), commonly to make injection molds for plastics.[46]
Powder and scrap
The powder is made by heating powdered petroleum coke above the temperature of graphitization, sometimes with minor modifications. The graphite scrap comes from pieces of unusable electrode material (in the manufacturing stage or after use) and lathe turnings, usually after crushing and sizing. Most synthetic graphite powder goes to carbon raising in steel (competing with natural graphite), with some used in batteries and brake linings. According to the USGS, US synthetic graphite powder and scrap production was 95,000 tonnes in 2001 (latest data).[10]
Neutron moderator
Special grades of synthetic graphite, such as Gilsocarbon,[47][48] also find use as a matrix and neutron moderator within nuclear reactors. Its low neutron cross-section also recommends it for use in proposed fusion reactors. Care must be taken that reactor-grade graphite is free of neutron absorbing materials such as boron, widely used as the seed electrode in commercial graphite deposition systems—this caused the failure of the Germans' World War II graphite-based nuclear reactors. Since they could not isolate the difficulty they were forced to use far more expensive heavy water moderators. Graphite used for nuclear reactors is often referred to as nuclear graphite.
Other uses
Graphite (carbon) fiber and carbon nanotubes are also used in carbon fiber reinforced plastics, and in heat-resistant composites such as reinforced carbon-carbon (RCC). Commercial structures made from carbon fiber graphite composites include fishing rods, golf club shafts, bicycle frames, sports car body panels, the fuselage of the Boeing 787 Dreamliner and pool cue sticks and have been successfully employed in reinforced concrete, The mechanical properties of carbon fiber graphite-reinforced plastic composites and grey cast iron are strongly influenced by the role of graphite in these materials. In this context, the term "(100%) graphite" is often loosely used to refer to a pure mixture of carbon reinforcement and resin, while the term "composite" is used for composite materials with additional ingredients.[49]
Modern smokeless powder is coated in graphite to prevent the buildup of static charge.
Graphite has been used in at least three radar absorbent materials. It was mixed with rubber in Sumpf and Schornsteinfeger, which were used on U-boat snorkels to reduce their radar cross section. It was also used in tiles on early F-117 Nighthawk stealth strike fighters.
Graphite composites are used as absorber for high-energy particles (e.g. in the LHC beam dump[50]).
Graphite mining, beneficiation, and milling

Large graphite specimen. Naturalis Biodiversity Center
Graphite is mined by both open pit and underground methods. Graphite usually needs beneficiation. This may be carried out by hand-picking the pieces of gangue (rock) and hand-screening the product or by crushing the rock and floating out the graphite. Beneficiation by flotation encounters the difficulty that graphite is very soft and "marks" (coats) the particles of gangue. This makes the "marked" gangue particles float off with the graphite, yielding impure concentrate. There are two ways of obtaining a commercial concentrate or product: repeated regrinding and floating (up to seven times) to purify the concentrate, or by acid leaching (dissolving) the gangue with hydrofluoric acid (for a silicate gangue) or hydrochloric acid (for a carbonate gangue).
In milling, the incoming graphite products and concentrates can be ground before being classified (sized or screened), with the coarser flake size fractions (below 8 mesh, 8–20 mesh, 20–50 mesh) carefully preserved, and then the carbon contents are determined. Some standard blends can be prepared from the different fractions, each with a certain flake size distribution and carbon content. Custom blends can also be made for individual customers who want a certain flake size distribution and carbon content. If flake size is unimportant, the concentrate can be ground more freely. Typical end products include a fine powder for use as a slurry in oil drilling and coatings for foundry molds, carbon raiser in the steel industry (Synthetic graphite powder and powdered petroleum coke can also be used as carbon raiser). Environmental impacts from graphite mills consist of air pollution including fine particulate exposure of workers and also soil contamination from powder spillages leading to heavy metal contamination of soil.
Occupational safety
People can be exposed to graphite in the workplace by breathing it in, skin contact, and eye contact.
United States
The Occupational Safety and Health Administration (OSHA) has set the legal limit (permissible exposure limit) for graphite exposure in the workplace as a time weighted average (TWA) of 15 million particles per cubic foot (1.5 mg/m3) over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of TWA 2.5 mg/m3 respirable dust over an 8-hour workday. At levels of 1250 mg/m3, graphite is immediately dangerous to life and health.[51]
Graphite recycling
The most common way of recycling graphite occurs when synthetic graphite electrodes are either manufactured and pieces are cut off or lathe turnings are discarded, or the electrode (or other) are used all the way down to the electrode holder. A new electrode replaces the old one, but a sizeable piece of the old electrode remains. This is crushed and sized, and the resulting graphite powder is mostly used to raise the carbon content of molten steel. Graphite-containing refractories are sometimes also recycled, but often not because of their graphite: the largest-volume items, such as carbon-magnesite bricks that contain only 15–25% graphite, usually contain too little graphite. However, some recycled carbon–magnesite brick is used as the basis for furnace-repair materials, and also crushed carbon–magnesite brick is used in slag conditioners. While crucibles have a high graphite content, the volume of crucibles used and then recycled is very small.
A high-quality flake graphite product that closely resembles natural flake graphite can be made from steelmaking kish. Kish is a large-volume near-molten waste skimmed from the molten iron feed to a basic oxygen furnace, and consists of a mix of graphite (precipitated out of the supersaturated iron), lime-rich slag, and some iron. The iron is recycled on site, leaving a mixture of graphite and slag. The best recovery process uses hydraulic classification (which utilizes a flow of water to separate minerals by specific gravity: graphite is light and settles nearly last) to get a 70% graphite rough concentrate. Leaching this concentrate with hydrochloric acid gives a 95% graphite product with a flake size ranging from 10 mesh down.
See also
- Acheson process
- Carbon fiber
- Carbon nanotube
- Diamond
- Exfoliated graphite nano-platelets
- Fullerene
- Graphene
- Graphite intercalation compound
- Intumescent
- Lonsdaleite
- Nuclear graphite
- Passive fire protection
- Plumbago drawing
- Pyrolytic carbon
References
^ Liquid method: pure graphene production. Phys.org (May 30, 2010).
^ Graphite. Mindat.org.
^ Graphite. Webmineral.com.
^ abc Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (1990). "Graphite". Handbook of Mineralogy (PDF). I (Elements, Sulfides, Sulfosalts). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209708..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "The types of coal". Coal - a traditional source of energy. OKD. 2012. Archived from the original on 2015-11-07. Retrieved 2017-08-24.
^ Sutphin, David M.; James D. Bliss (August 1990). "Disseminated flake graphite and amorphous graphite deposit types; an analysis using grade and tonnage models". CIM Bulletin. 83 (940): 85–89.
^ abcdef graphite. Encyclopædia Britannica Online.
^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "highly oriented pyrolytic graphite".
^ "Mineral Commodity Summaries 2017" (PDF).
^ abcdefghijk "Graphite Statistics and Information". USGS. Retrieved 2009-09-09.
^ Delhaes, P. (2001). Graphite and Precursors. CRC Press. ISBN 90-5699-228-7.
^ Lipson, H.; Stokes, A. R. (1942). "A New Structure of Carbon". Nature. 149 (3777): 328. Bibcode:1942Natur.149Q.328L. doi:10.1038/149328a0.
^ Wyckoff, W.G. (1963). Crystal Structures. New York, London: John Wiley & Sons. ISBN 0-88275-800-4.
^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Rhombohedral graphite".
^ Hanaor, D.; Michelazzi, M.; Leonelli, C.; Sorrell, C.C. (2011). "The effects of firing conditions on the properties of electrophoretically deposited titanium dioxide films on graphite substrates". Journal of the European Ceramic Society. 31 (15): 2877–2885. arXiv:1303.2757. doi:10.1016/j.jeurceramsoc.2011.07.007.
^ Deprez, N.; McLachlan, D. S. (1988). "The analysis of the electrical conductivity of graphite conductivity of graphite powders during compaction". Journal of Physics D: Applied Physics. Institute of Physics. 21 (1): 101–107. Bibcode:1988JPhD...21..101D. doi:10.1088/0022-3727/21/1/015.
^ Lavrakas, Vasilis (1957). "Textbook errors: Guest column. XII: The lubricating properties of graphite". Journal of Chemical Education. 34 (5): 240. Bibcode:1957JChEd..34..240L. doi:10.1021/ed034p240.
^ Watanabe, N.; Hayakawa, H.; Yoshimoto, O.; Tojo, T. (2000). "The lubricating properties of graphite fluoride composites under both atmosphere and high vacuum condition". FY2000 Ground – based Research Announcement for Space Utilization Research Report.
^ Yen, Bing; Schwickert, Birgit (2004). Origin of low-friction behavior in graphite investigated by surface x-ray diffraction, SLAC-PUB-10429 (PDF) (Report). Retrieved March 15, 2013.
^ Galvanic Corrosion. keytometals.com
^ "ASM Tech Notes – TN7-0506 – Galvanic Corrosion" (PDF). Atlas Specialty Metals. Archived from the original (PDF) on 2009-02-27.
^ Jones, Rick (USAF-Retired) Better Lubricants than Graphite. graflex.org
^ "Weapons Lubricant in the Desert". September 16, 2005. Archived from the original on 2007-10-15. Retrieved 2009-06-06.
^ "Good Engineering Practice/Corrosion". Retrieved 2009-06-06.
^ Boardman, John. "The Neolithic-Eneolithic Period". The Cambridge ancient history, Volume 3, Part 1 (PDF). pp. 31–32. ISBN 0521224969. Archived from the original (PDF) on 25 February 2013.
^ Norgate, Martin and Norgate, Jean; Geography Department, Portsmouth University (2008). "Old Cumbria Gazetteer, black lead mine, Seathwaite". Retrieved 2008-05-19.CS1 maint: Multiple names: authors list (link)
^ Wainwright, Alfred (2005). A Pictorial Guide to the Lakeland Fells, Western Fells. London: Frances Lincoln. ISBN 0-7112-2460-9.
^ The Statutes at Large: From the ... Year of the Reign of ... to the ... Year of the Reign of . 1764. p. 415.
^ Dixon Ticonderoga Company http://www.dixonusa.com/history.html. Retrieved 6 April 2018. Missing or empty|title=(help)
^ Nguyen, Ahn (2003). Colloidal Science of Flotation. p. 11. ISBN 0824747828.
^ Ibid.
^ Cirkel, Fritz (1907). Graphite its Properties, Occurrence, Refining and Uses. Ottawa: Canadian Department of Mines. p. passim. Retrieved 6 April 2018.
^ Electro-Plating on Non-Metallic Substances. Spons' Workshop Receipts. Vol. II: Dyeing to Japanning. Spon. 1921. p. 132.
^ Evans, John W. (1908). "V.— the Meanings and Synonyms of Plumbago". Transactions of the Philological Society. 26 (2): 133–179. doi:10.1111/j.1467-968X.1908.tb00513.x.
^ Widenmann, Johann Friedrich Wilhelm (1794). Handbuch des oryktognostischen Theils der Mineralogie: Mit einer Farbentabelle und einer Kupfertafel. Crusius. p. 653.
^
Scheele, C. W. K. (1779). "Versuche mit Wasserbley; Molybdaena". Svenska vetensk. Academ. Handlingar. 40: 238.
^ ab "Electric Graphite Growing Demand From Electric Vehicles & Mobile Electronics" (PDF). galaxycapitalcorp.com. July 20, 2011.
^ Harper, Douglas. "graphite". Online Etymology Dictionary.
^ Ritter, Steve (October 15, 2001). "Pencils & Pencil Lead". American Chemical Society.
^ "The History of the Pencil". University of Illinois at Urbana–Champaign.
^ "Module 6: Media for 2-D Art" (PDF). Saylor.org. Retrieved 2 April 2012.
^ True color/appearance of the "Graphite, or Smokebox colors. List.nwhs.org. Retrieved on 2013-04-15.
^ Emery, Nicolas; Hérold, Claire; Marêché, Jean-François; Lagrange, Philippe (2008). "Synthesis and superconducting properties of CaC6". Sci. Technol. Adv. Mater. 9 (4): 044102. Bibcode:2008STAdM...9d4102E. doi:10.1088/1468-6996/9/4/044102. PMC 5099629. PMID 27878015.
^ Acheson, E. G. "Manufacture of Graphite", U.S. Patent 568,323, issued September 29, 1896.
^ Lapshin, R. V. (1998). "Automatic lateral calibration of tunneling microscope scanners" (PDF). Review of Scientific Instruments. 69 (9): 3268–3276. Bibcode:1998RScI...69.3268L. doi:10.1063/1.1149091.
^ Hugh O. Pierson – Handbook of Carbon, Graphite, Diamonds and Fullerenes: Processing, Properties and Applications – Noyes Publication
ISBN 0-8155-1339-9
^ Arregui-Mena, J.D.; Bodel, W.; et al. (2016). "Spatial variability in the mechanical properties of Gilsocarbon". Carbon. 110: 497–517. doi:10.1016/j.carbon.2016.09.051.
^ Arregui Mena, J.D.; et al. (2018). "Characterisation of the spatial variability of material properties of Gilsocarbon and NBG-18 using random fields". Journal of Nuclear Materials. 511: 91–108. doi:10.1016/j.jnucmat.2018.09.008.
^ Cooper, Jeff. What is the best material for a tennis racquet?. tennis.about.com
^ Yurkewicz, Katie. "Protecting the LHC from itself" (PDF). Symmetry Magazine.
^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Graphite (natural)". www.cdc.gov. Retrieved 2015-11-03.
Further reading
C.Michael Hogan; Marc Papineau; et al. (December 18, 1989). Phase I Environmental Site Assessment, Asbury Graphite Mill, 2426–2500 Kirkham Street, Oakland, California, Earth Metrics report 10292.001 (Report).
Klein, Cornelis; Cornelius S. Hurlbut, Jr. (1985). Manual of Mineralogy: after Dana (20th ed.). ISBN 0-471-80580-7.
Taylor, Harold A. (2000). Graphite. Financial Times Executive Commodity Reports. London: Mining Journal Books ltd. ISBN 1-84083-332-7.
Taylor, Harold A. (2005). Graphite. Industrial Minerals and Rocks (7th ed.). Littleton, CO: AIME-Society of Mining Engineers. ISBN 0-87335-233-5.
External links
| Wikimedia Commons has media related to Graphite. |
- Battery Grade Graphite
- Graphite at Minerals.net
- Mineral galleries
Mineral & Exploration – Map of World Graphite Mines and Producers 2012- Mindat w/ locations
- giant covalent structures
- The Graphite Page
Video lecture on the properties of graphite by Prof. M. Heggie, University of Sussex
- CDC – NIOSH Pocket Guide to Chemical Hazards






