Caesium iodide
 CsI crystal | |
 Scintillating CsI crystal | |
 Crystal structure | |
| Names | |
|---|---|
IUPAC name Caesium iodide | |
| Other names Cesium iodide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.029.223 |
EC Number | 232-145-2 |
PubChem CID |
|
RTECS number | FL0350000 |
UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | CsI |
Molar mass | 259.809 g/mol[2] |
| Appearance | white crystalline solid |
Density | 4.51 g/cm3[2] |
Melting point | 632 °C (1,170 °F; 905 K)[2] |
Boiling point | 1,280 °C (2,340 °F; 1,550 K)[2] |
Solubility in water | 848 g/L (25 °C)[2] |
Magnetic susceptibility (χ) | -82.6·10−6 cm3/mol[3] |
Refractive index (nD) | 1.9790 (0.3 µm) 1.7873 (0.59 µm) 1.7694 (0.75 µm) 1.7576 (1 µm) 1.7428 (5 µm) 1.7280 (20 µm)[4] |
| Structure | |
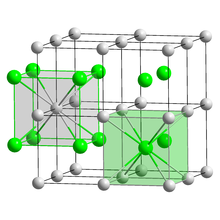
Crystal structure | CsCl, cP2 |
Space group | Pm3m, No. 221[5] |
Lattice constant | a = 0.4503 nm |
Lattice volume (V) | 0.0913 nm3 |
Formula units (Z) | 1 |
Coordination geometry | Cubic (Cs+) Cubic (I−) |
| Thermochemistry | |
Heat capacity (C) | 52.8 J/mol·K[6] |
Std molar entropy (S | 123.1 J/mol·K[6] |
Std enthalpy of formation (ΔfH | −346.6 kJ/mol[6] |
Gibbs free energy (ΔfG˚) | -340.6 kJ/mol[6] |
| Hazards | |
GHS pictograms |    |
GHS signal word | Warning |
GHS hazard statements | H315, H317, H319, H335 |
GHS precautionary statements | P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+312, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P330, P332+313, P333+313, P337+313, P362, P363, P391, P403+233 |
Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 2386 mg/kg (oral, rat)[1] |
| Related compounds | |
Other anions | Caesium fluoride Caesium chloride Caesium bromide Caesium astatide |
Other cations | Lithium iodide Sodium iodide Potassium iodide Rubidium iodide Francium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Caesium iodide or cesium iodide (chemical formula CsI) is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.[7]
Contents
1 Synthesis and structure
2 Properties
3 Applications
4 References
5 Cited sources
Synthesis and structure
Monatomic caesium halide wires grown inside double-wall carbon nanotubes.[8]
Bulk caesium iodide crystals have the cubic CsCl crystal structure, but the structure type of nanometer-thin CsI films depends on the substrate material – it is CsCl for mica and NaCl for LiF, NaBr and NaCl substrates.[9]
Caesium iodide atomic chains can be grown inside double-wall carbon nanotubes. In such chains I atoms appear brighter than Cs atoms in electron micrographs despite having a smaller mass. This difference was explained by the charge difference between Cs atoms (positive), inner nanotube walls (negative) and I atoms (negative). As a result, Cs atoms are attracted to the walls and vibrate more strongly than I atoms, which are pushed toward the nanotube axis.[8]
Properties
| Т (°C) | 0 | 10 | 20 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (wt%) | 30.9 | 37.2 | 43.2 | 45.9 | 48.6 | 53.3 | 57.3 | 60.7 | 63.6 | 65.9 | 67.7 | 69.2 |
Applications
An important application of caesium iodide crystals, which are scintillators, is electromagnetic calorimetry in experimental particle physics. Pure CsI is a fast and dense scintillating material with relatively low light yield that increases significantly with cooling.[11] It shows two main emission components: one in the near ultraviolet region at the wavelength of 310 nm and one at 460 nm. The drawbacks of CsI are a high temperature gradient and a slight hygroscopicity.
Caesium iodide is used as a beamsplitter in Fourier transform infrared (FTIR) spectrometers. It has a wider transmission range than the more common potassium bromide beamsplitters, extending its working range into the far infrared. However, optical-quality CsI crystals are very soft and hard to cleave or polish. They should also be coated (typically with germanium) and stored in a desiccator, to minimize interaction with atmospheric water vapors.[12]
In addition to image intensifier input phosphors, caesium iodide is often also used in medicine as the scintillating material in flat panel x-ray detectors.[13]
References
| Wikimedia Commons has media related to Caesium iodide. |
^ ab Cesium iodide. U.S. National Library of Medicine
^ abcde Haynes, p. 4.57
^ Haynes, p. 4.132
^ Haynes, p. 10.240
^ Huang, Tzuen-Luh; Ruoff, Arthur L. (1984). "Equation of state and high-pressure phase transition of CsI". Physical Review B. 29 (2): 1112. Bibcode:1984PhRvB..29.1112H. doi:10.1103/PhysRevB.29.1112..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcd Haynes, p. 5.10
^ Kowalski, M. P.; Fritz, G. G.; Cruddace, R. G.; Unzicker, A. E.; Swanson, N. (1986). "Quantum efficiency of cesium iodide photocathodes at soft x-ray and extreme ultraviolet wavelengths". Applied Optics. 25 (14): 2440. Bibcode:1986ApOpt..25.2440K. doi:10.1364/AO.25.002440. PMID 18231513.
^ ab Senga, Ryosuke; Komsa, Hannu-Pekka; Liu, Zheng; Hirose-Takai, Kaori; Krasheninnikov, Arkady V.; Suenaga, Kazu (2014). "Atomic structure and dynamic behaviour of truly one-dimensional ionic chains inside carbon nanotubes". Nature Materials. 13 (11): 1050. Bibcode:2014NatMa..13.1050S. doi:10.1038/nmat4069. PMID 25218060.
^ Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487. doi:10.1107/S0365110X51001641.
^ Haynes, p. 5.191
^ Mikhailik, V.; Kapustyanyk, V.; Tsybulskyi, V.; Rudyk, V.; Kraus, H. (2015). "Luminescence and scintillation properties of CsI: A potential cryogenic scintillator". Physica Status Solidi B. 252 (4): 804–810. arXiv:1411.6246. Bibcode:2015PSSBR.252..804M. doi:10.1002/pssb.201451464.
^ Sun, Da-Wen (2009). Infrared Spectroscopy for Food Quality Analysis and Control. Academic Press. pp. 158–. ISBN 978-0-08-092087-0.
^ Lança, Luís; Silva, Augusto (2012). "Digital Radiography Detectors: A Technical Overview". Digital Imaging Systems for Plain Radiography (PDF). Springer. doi:10.1007/978-1-4614-5067-2_2. ISBN 978-1-4614-5066-5.
Cited sources
Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1439855110.